| ProFootballLogic | ||||||||||
| ARTICLES | TEAMS | STATS | RATINGS | STANDINGS | GAMES | SCHEDULE | PLAYERS | METHOD | SPORTS | |
The Science of Football Deflation
By Michael Gertz
Tuesday, January 27, 2015
In the wake of the deflation scandal from the AFC Championship, Bill Belichick and some others have now presented some possible scenarios that they believe could compromise the NFL's ball pressure measurements. With that in mind, let's look at the actual thermodynamic principles that govern footballs to better understand the situation.
The Measurement Process
Before we examine how the pressure of a football could change, we must keep in mind how the pressure is measured. All NFL measurements are taken indoors at room temperature. That means that if the NFL lets balls return to equilibrium indoors, none of the following effects would significantly impact the measurements.
Whether the NFL always takes the time to ensure balls do reach equilibrium has yet to be reported. After examining different scenarios of ball pressure change, we will then estimate how long it would take the effects to reach equilibrium back at room temperature.
Effects of Temperature on Football Pressure
Other than changing the amount of air, temperature is the about the only thing that can possibly affect the air pressure inside a football in a non-negligible way. Air follows the Ideal Gas Law, which states that
P * V = n * R * T
where:
P = Pressure
V = Volume
n = Amount of molecules
R = Gas constant
T = Temperature
R is simply a constant that depends on the units of measurement. n is the amount of air inside the football, so it is constant if the ball is not inflated or deflated. V is the volume inside the ball, which could theoretically increase with pressure (like a balloon) or with temperature due to the leather expanding (like an asphalt road cracking), but both of these effects are likely negligible compared to the pressure changes in footballs. Therefore we are left with a simple law that pressure is directly proportional to temperature for the air inside a football.
The only caveat is that we must measure both quantities on an absolute scale. This means using Kelvin units for temperature and accounting for atmospheric pressure, since ball pressure is actually measured as only the amount of pressure above atmospheric pressure.
The "Rub Down" Effect
While there are conflicting reports as to whether balls are prepped by rubbing or brushing during the week of practice prior to a game or just before they are handed to officials, we will examine the possibility that they could have been prepared just before being inspected by officials.
Bill Belichick has stated that their tests showed that a rubbed down ball can increase in pressure by 1 psi. Although Bill Nye disputed that possibility, we have been able to confirm it in our own test. Vigorous friction on a football can make it warm to the touch, and heat the air inside enough to account for a 1 psi increase in pressure.
Using the equation above, 72 degrees F for room temperature, and an atmospheric pressure of 14.59 psi measured in the Boston area at the start of the AFC Championship, a ball with only an initial 11.5 psi at room temperature would measure at an acceptable 12.5 psi if the measurement occurred while the air in the ball was still at about 92 degrees.
The Outdoor Temperature Effect
Although both the air in the ball and outside the ball dropped from about 72 to 48 degrees during the game, the colder temperature would not affect the pressure of the outdoor air the same way it would the air inside the ball, because the indoor and outdoor air at the stadium are not closed systems. The difference in air pressure between indoors and outdoors is constantly being equilized when doors are opened or cracks exist.
Therefore the air pressure indoors for inspections and outdoors during the game would be about the same. This doesn't violate the Ideal Gas Law, because molecules are free to leave a heated building and move outdoors. So for atmospheric pressure, the number of molecules offsets the temperature difference between indoor and outdoor air while the pressures remain the same.
Continuing to use the most extreme case possible where the NFL takes measurements immediately, a ball that had an original room temperature pressure of 11.5 psi would drop to 10.3 psi while outdoors at 48 degrees. This is a larger drop than even the 0.5 psi drop Belichick mentioned due to outdoor temperatures.
The Changing Atmospheric Pressure Effect
Over the course of the 1st half of the AFC Championship, the atmospheric pressure in the Boston area dropped from about 14.59 to 14.53 psi. Because as we stated above that indoor air pressure would likely quickly equal outdoor atmospheric pressure, this is the one factor that could disrupt the NFL's measurements even if they did wait long enough for the balls to return to room temperature before taking all measurements.
That difference of 0.06 psi would directly increase any measurement taken at halftime compared to pre-game by that exact amount. However, that change is so small that it has little bearing on the situation overall.
How Fast Footballs Change Temperature
So far we have presented a scenario in which, if the NFL were terribly misguided and took all measurements of footballs immediately, they could have ended up with a football that was measured pre-game at 12.5 psi at 92 degrees and later measured at halftime at 10.4 psi at 48 degrees. That is a difference of 2.1 psi, which is about equal to the reported missing 2 psi.
However, when we examine the plausibility of such a scenario existing based on how quickly footballs change temperature, it begins to look like a near impossiblity. In our own test, a football and the air inside it can undergo even about a 40 degree F temperature change, with the expected associated changes in pressure, in a matter of only about 20 minutes after being placed in the different environment.
It makes sense that footballs would change temperature relatively quickly compared to other objects. This is because they have a small mass, and the surface area to volume ratio of the ball's leather is very high due to the shape.
The temperature changes in question are only 20 and then 24 degrees F each. Considering the officials were testing at least 12 balls at both the pre-game and halftime inspections all indoors, even if they were lightning quick with their measurements, it is virtually impossible for the average ball to have actually been measured before having already lost at least half of its temperature difference.
Even in the most extreme case imaginable, the pressure difference would reaslistically be no more than 1 psi rather than the 2.1 psi associated with instantaneous measurements. Keep in mind, even that is assuming that the NFL had a flawed process to begin with. If there is any time delay in either inspection at all, whether purposefully or accidentally, the thermodynamic effects would dimish almost entirely.
Realistically, there are only 2 possible explanations to Deflategate. Either the Patriots deflated the balls after the inspection, or the first inspection never properly took place. And according to all reports so far, the NFL is confident that the initial inspection was properly done.
The Bigger Picture
The level of punishment given to the New England Patriots should they be found guilty of tampering with balls is a matter of opinion, but there are some relevant factors that many people have failed to consider. While no one would argue that deflated balls were the reason the Patriots won the AFC Championship game, punishment in all its forms should be relative to intent rather than effect. The reason intent is important is that the NFL can't have a 100% success rate of catching rule breakers. It seems unlikely that a team deflating its footballs would be caught the very first time they do it.
Therefore, it seems likely that if found guilty in this instance, the Patriots had previously gotten away with this same offense several times, if not for years. Further, if they are now found to have willingly broken rules twice under Belichick and Brady, it seems likely that they have also gotten away with other types of infractions that have yet to be discovered. While deflated balls were unlikely to have determined the outcome of the AFC Championship, they or different undiscovered infractions very well may have been a crucial factor in several closer games in the past.
How Much Ball Pressure Varies During Games
Let's now examine the range of ball pressures that are possible during an NFL game even when properly inflated at room temperature. We've seen that even a 48 degree F game can have a noticeable effect on ball pressure, so let's explore the full range of weather possibilities.
Below is a graph representing pressures that legal game balls initially measured between 12.5 and 13.5 psi at 72 degrees F would reach outdoors during a game due to temperature changes. The thick red "High" line represents a ball initially inflated to 13.5 psi at room temperature, and the thick blue "Low" line represents a ball initially inflated to 12.5 psi at room temperature. If the NFL halftime measurements were correct and the Patriots balls were around 10.5 psi at room temperature, it would mean that they were about 9.4 psi during the game rather than the 11.3 they should have been.
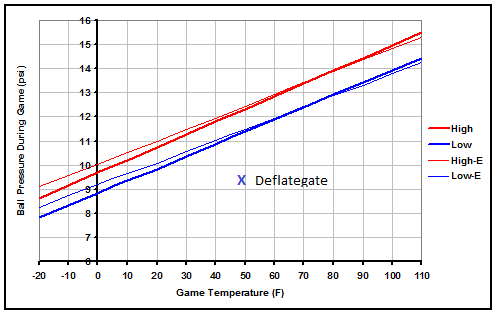
Game ball pressure does vary considerably with temperature, and much wider than the initial range of acceptable room temperature pressures. However, in very cold weather the effects of cold fingers and stiff cold footballs apparently offset the pressure difference, as very cold balls are still often referred to as "harder". In the most extreme of cold weather games, football pressure could actually drop a full 4 psi to below 9 psi. While that difference is easily noticeable, 9 psi is still in the range of where amateur balls are often inflated.
Unless that same effect of the football being more or less stiff in cold or hot weather is consistent throughout the temperature range, teams would actually be wise to choose their pre-game ball pressure relative to the weather. For instance, a team that likes 12.5 psi balls would actually have to prepare about 13.5 psi balls to get 12.5 psi balls during a 50 degree game, while a team that likes 13.5 psi balls would have to prepare about 12.5 psi balls to get 13.5 psi balls during a 90 degree game.
The Effect of Elevation
Atmospheric pressure varies considerably with elevation. But because ball pressure is measured at 13 psi relative to atmospheric pressure, elevation has no effect whatsoever on the feel of a football or its pressure measurement while it is at constant temperature and elevation. However when a football is taken from room temperature outdoors to a different temperature at elevation, the fact that it has a slightly lower absolute pressure means that it's pressure will change slightly less than a sea level ball would at a given temperature.
In the above graph, the thin lines represent a game played at an elevation of about 5,200 ft above sea level like in Denver, the only NFL stadium that is located significantly above sea level. Overall, the effects of elevation on game ball pressure is minimal regardless of the temperature. However, the lower atmospheric pressure does reduce the drag on a thrown or kicked ball slightly which can impact a game.
While simply being at elevation has no real impact on ball pressure so long as the balls were also measured at elevation, moving a ball from low elevation to high elevation will increase its pressure (or decrease if moved from high to low elevation). Though the ball's absolute pressure remains the same, the decrease in atmospheric pressure creates a larger relative pressure difference, and that relative difference is what is being measured by a pressure gauge.
The math is much simpler for calculating elevation pressure changes. The difference in atmospheric pressure is simply added to the ball's pressure. Below is a table of how moving a standard 13 psi football at sea level to different elevations would change its air pressure.
| Effect of Elevation Change on Ball Filled at Sea Level | |||
|---|---|---|---|
| Location | Elevation (ft) | Atmospheric Pressure (psi) | Football Pressure (psi) |
| Sea Level | 0 | 14.7 | 13.0 |
| Glendale, AZ | 1,152 | 14.1 | 13.6 |
| Denver | 5,280 | 12.1 | 15.6 |
| Airplane | 7,000(eq) | 11.3 | 16.4 |
| Mt. Everest | 29,029 | 4.6 | 24.1 |
Airplanes are kept pressurized at a level equivalent to about 7,000 ft of elevation. Bottom line: make sure to deflate your sports balls before taking them on an airplane or a ski trip! Although the pressure is temporary, they could get overinflated by over 3 psi and their integrity could be permanently compromised. The home of this year's Super Bowl in Arizona is the 2nd highest NFL stadium, but even it is less than 1/4th the elevation of Denver. For the record, Wyoming has the highest Division I FBS football stadium at 7,215 ft.
| Recent Articles |
|---|
| If 2021 Had 16 Games - 1/10/22 |
| Wk 18 Playoff Scenarios 2021 - 1/8/22 |
| Wk 17 Playoff Scenarios 2020 - 1/1/21 |
| Wk 17 Playoff Scenarios 2019 - 12/27/19 |
| 2 Week Playoff Scenarios 2019 - 12/21/19 |
| 3 Week Playoff Tiebreakers 2019 - 12/11/19 |
| NFL Injury Point Value - 6/18/19 |
| How Teams Value Draft Picks - 4/25/19 |
| Analyzing The Zion Injury - 3/21/19 |
| Week 17 Playoff Scenarios 2018 - 12/27/18 |
| BUF | MIA | NE | NYJ | BAL | CIN | CLE | PIT | HOU | IND | JAC | TEN | DEN | KC | LV | LAC | |||||||||||||
| DAL | NYG | PHI | WAS | CHI | DET | GB | MIN | ATL | CAR | NO | TB | ARI | LAR | SF | SEA | |||||||||||||
| ProFootballLogic.com welcomes questions, requests, and error reports by email to contact@profootballlogic.com | ||||||||||||||||||||||||||||
| Privacy Policy | ||||||||||||||||||||||||||||
| Copyright © 2025 ProFootballLogic.com. All Rights Reserved. | ||||||||||||||||||||||||||||